Is a Hydrogen Revolution Possible?
10 min read
Many energy specialists see as a key path in the . While a great deal of research and many technological advances will be needed for this vision to become reality, a clear movement is underway in a number of the world's major countries.

© BLAISE BERNARD / TotalEnergies - Close up of a hydrogen nozzle in a service station.
Jules Verne’s Prescient Vision
The possibility of using hydrogen as an has been understood since the 19th century. In his novel “The Mysterious Island”, French author Jules Verne anticipated an energy revolution through the character of Cyrus Smith, an engineer: “Yes, my friends, I believe that water will one day be employed as , that hydrogen and oxygen which constitute it, used singly or together, will furnish an inexhaustible source of and light, of an intensity of which is not capable.”
In the 1840's, hydrogen from coal gas was used for public lighting and town gas. It gained new popularity in the second half of the 20th century with the space program, as researchers looked for ways to generate energy autonomously onboard satellites and to launch rockets.
Initial Applications
What has changed since the early 19th century is the dynamic that has spurred on teams of researchers worldwide and already resulted in the first industrial and consumer applications in countries such as Japan, South Korea, the United States and Germany 1. The first hydrogen cars have already reached the market; stationary installations the size of a wardrobe are starting to supply and heat to buildings and stores; and some buses, trucks and lift trucks are already running on hydrogen.
While hydrogen has mainly been used until now in ammonia production and oil refining, it seems destined for major development as an energy carrier.
The Problem of Production
Hydrogen’s main drawback is that it needs to be produced. It is not a source like oil, gas, or wind, but rather what is known as an “energy carrier”, like electricity and heat.
To be sure, natural hydrogen seeps have been detected at the bottom of the ocean or in the center of certain continental basins, notably on the Russian plains. Hydrogen has also been found to occur naturally in gas sludge in Mali and the United States, where efforts have begun to capture it.
But the possibility of operating a hydrogen field is something that lies far in the future – if at all – and for now, hydrogen has to be produced by separating it from other elements such as carbon or the oxygen in water. This means that hydrogen is a renewable resource. The level of CO2 emissions depends entirely on the production method. Most hydrogen today is produced from fossil fuels, but biomass gasification and electrolysis of water appear to be real possibilities, making hydrogen a potentially “clean” fuel.
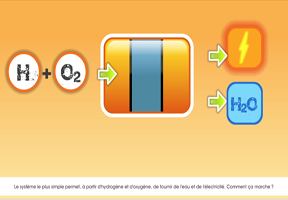
In use, hydrogen does not generate any CO2 either. In a , it produces electricity and gives off water and heat.
Storage and Cost
While hydrogen has a very high mass (it contains three times more energy per unit of weight than ), it is very difficult to store and distribute. It takes energy to liquefy or compress hydrogen, and because the gas is very light, its transportation is very inefficient by unit of volume (15 times less efficient than oil and three times less than natural gas).
Excluding distribution, the ex-plant cost of hydrogen produced via steam reforming of methane stands at around €1.50 per kilogram of H2. The price is much higher for hydrogen generated by industrial electrolyzers, at between €5 and €30 per kilogram, depending on the price of electricity. As for the price at the pump for future hydrogen cars (i.e., after transportation and compression), a target of €10 per kilogram by 2020 is considered necessary to convince consumers, given that a vehicle can drive for more than 100 kilometers on one kilogram of hydrogen.
Sources :
- CEA (in French only)