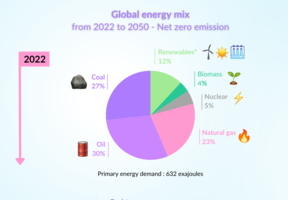
How Do Hydrogen Cars Work?
2 min read
The cell generates from gas, meaning that hydrogen cars can make their own electricity to their engines.
How do hydrogen cars work?
Hydrogen cars are similar to electric cars.
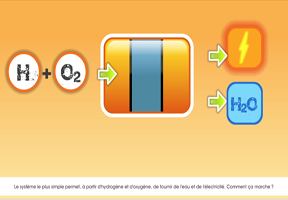
But instead of being powered by a battery that is used to store electricity, hydrogen cars make their own electricity using a . How fuel cells work: oxygen and hydrogen react to produce an electrical current.
The hydrogen is directed to an electrode called an anode. Its electrons are released and forced through an external circuit, generating direct current.
The resulting ions pass through the electrolyte to the cathode, where they recover the electrons and combine with oxygen to form water.
The water is then released through the car’s exhaust.
Hydrogen limitations:
- Hydrogen is a light , so it is difficult to store – but less so than electricity.
- The chemical reaction needs to be accelerated by a catalyst: platinum, a rare and therefore expensive metal.
- Hydrogen does not exist in its pure form on Earth, so it must be produced by splitting water. A costly process but one that does not emit
.
That said, hydrogen cars have all the advantages of electric vehicles:
- No CO2 emissions or exhaust pollutants, plus a longer range than current electric vehicles.
So this technology’s development will largely depend on whether electric vehicles can extend their range.
WORDS ON DIARAMS: “Hydrogen tank” “Oxygen supply” “Fuel cell” “Motor” “Exhaust” “– Anode” “+ Cathode” “Electrical current (electrons)” “Water vapor” “Electrolyte”
View more videos here